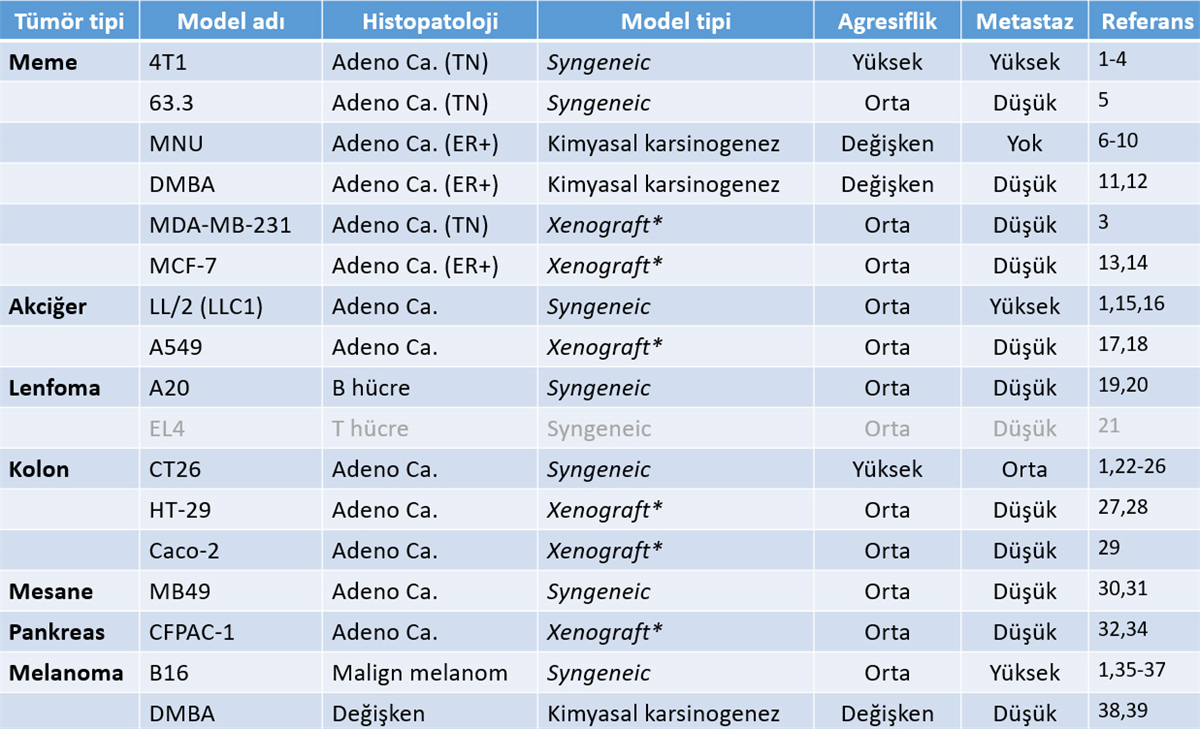
1. Lechner, M.G., et al., Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J Immunother, 2013. 36(9): p. 477-89.
2. Pulaski, B.A. and S. Ostrand-Rosenberg, Mouse 4T1 breast tumor model. Curr Protoc Immunol, 2001. Chapter 20: p. Unit 20 2.
3. Yang, S., J.J. Zhang, and X.Y. Huang, Mouse models for tumor metastasis. Methods Mol Biol, 2012. 928: p. 221-8.
4. Turhan, N., et al., Co-existence of Echinococcus granulosus infection and cancer metastasis in the liver correlates with reduced Th1 immune responses. Parasite Immunol, 2015. 37(1): p. 16-22.
5. Lachman, L.B., et al., DNA vaccination against neu reduces breast cancer incidence and metastasis in mice. Cancer Gene Ther, 2001. 8(4): p. 259-68.
6. Tsubura, A., et al., Review: Animal models of N-Methyl-N-nitrosourea-induced mammary cancer and retinal degeneration with special emphasis on therapeutic trials.In Vivo, 2011. 25(1): p. 11-22.
7. Pazos, P., et al., Mammary carcinogenesis induced by N-methyl-N-nitrosourea (MNU) and medroxyprogesterone acetate (MPA) in BALB/c mice. Breast Cancer Res Treat, 1992. 20(2): p. 133-8.
8. Dolen, Y., et al., Granulocytic subset of myeloid derived suppressor cells in rats with mammary carcinoma. Cell Immunol, 2015. 295(1): p. 29-35.
9. Esendagli, G., et al., Primary tumor cells obtained from MNU-induced mammary carcinomas show immune heterogeneity which can be modulated by low-efficiency transfection of CD40L gene. Cancer Biol Ther, 2009. 8(2): p. 136-42.
10. Esendagli, G., et al., Coexistence of different tissue tumourigenesis in an N-methyl-N-nitrosourea-induced mammary carcinoma model: a histopathological report in Sprague-Dawley rats. Lab Anim, 2009. 43(1): p. 60-4.
11. Abba MC, Zhong Y, Lee J, Kil H, Lu Y, Takata Y, Simper MS, Gaddis S, Shen J, Aldaz CM. DMBA induced mouse mammary tumors display high incidence of activating Pik3caH1047 and loss of function Pten mutations. Oncotarget. 2016 Sep 27;7(39):64289-64299.
12. Liu Z, Kundu-Roy T, Matsuura I, Wang G, Lin Y, Lou YR, Barnard NJ, Wang XF, Huang MT, Suh N, Liu F. Carcinogen 7,12-dimethylbenz[a]anthracene-induced mammary tumorigenesis is accelerated in Smad3 heterozygous mice compared to Smad3 wild type mice. Oncotarget. 2016 Oct 4;7(40):64878-64885.
13. Kubota, T., et al., Human breast carcinoma (MCF-7) serially transplanted into nude mice. Jpn J Surg, 1983. 13(4): p. 381-4.
14. Zhai, Y.F., et al., Growth of MCF-7 human breast carcinoma in severe combined immunodeficient mice: growth suppression by recombinant interleukin-2 treatment and role of lymphokine-activated killer cells. Cancer Immunol Immunother, 1992. 35(4): p. 237-45.
15. Bertram, J.S. and P. Janik, Establishment of a cloned line of Lewis Lung Carcinoma cells adapted to cell culture. Cancer Lett, 1980. 11(1): p. 63-73.
16. Unver, N., et al., CXCL7-induced macrophage infiltration in lung tumor is independent of CXCR2 expression: CXCL7-induced macrophage chemotaxis in LLC tumors.Cytokine, 2015. 75(2): p. 330-7.
17. Kang, Y., et al., Development of an orthotopic transplantation model in nude mice that simulates the clinical features of human lung cancer. Cancer Sci, 2006. 97(10): p. 996-1001.
18. Kang, Y., et al., Proliferation of human lung cancer in an orthotopic transplantation mouse model. Exp Ther Med, 2010. 1(3): p. 471-475.
19. Donnou, S., et al., Murine models of B-cell lymphomas: promising tools for designing cancer therapies. Adv Hematol, 2012. 2012: p. 701704.
20. Kim, K.J., et al., Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol, 1979. 122(2): p. 549-54.
21. Komohara, Y., et al., Delayed growth of EL4 lymphoma in SR-A-deficient mice is due to upregulation of nitric oxide and interferon-gamma production by tumor-associated macrophages. Cancer Sci, 2009. 100(11): p. 2160-6.
22. Donigan, M., et al., A metastatic colon cancer model using nonoperative transanal rectal injection. Surg Endosc, 2010. 24(3): p. 642-7.
23. Donigan, M., et al., Novel murine model for colon cancer: non-operative trans-anal rectal injection. J Surg Res, 2009. 154(2): p. 299-303.
24. Kishimoto, H., et al., Development of a clinically-precise mouse model of rectal cancer. PLoS One, 2013. 8(11): p. e79453.
25. Mittal, V.K., J.S. Bhullar, and K. Jayant, Animal models of human colorectal cancer: Current status, uses and limitations. World J Gastroenterol, 2015. 21(41): p. 11854-61.
26. Zigmond, E., et al., Utilization of murine colonoscopy for orthotopic implantation of colorectal cancer. PLoS One, 2011. 6(12): p. e28858.
27. Jin, H., et al., A superficial colon tumor model involving subcutaneous colon translocation and orthotopic transplantation of green fluorescent protein-expressing human colon tumor. Tumour Biol, 2011. 32(2): p. 391-7.
28. McIntyre, R.E., et al., Mouse models of colorectal cancer as preclinical models. Bioessays, 2015. 37(8): p. 909-20.
29. Fogh, J., J.M. Fogh, and T. Orfeo, One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst, 1977. 59(1): p. 221-6.
30. Loskog, A., et al., Optimization of the MB49 mouse bladder cancer model for adenoviral gene therapy. Lab Anim, 2005. 39(4): p. 384-93.
31. Dobek, G.L. and W.T. Godbey, An orthotopic model of murine bladder cancer. J Vis Exp, 2011(48).
32. Deer, E.L., et al., Phenotype and genotype of pancreatic cancer cell lines. Pancreas, 2010. 39(4): p. 425-35.
33. Qu, C.F., et al., In vivo and in vitro inhibition of pancreatic cancer growth by targeted alpha therapy using 213Bi-CHX.A”-C595. Cancer Biol Ther, 2005. 4(8): p. 848-53.
34. Ozturk, K., et al., Effective targeting of gemcitabine to pancreatic cancer through PEG-cored Flt-1 antibody-conjugated dendrimers. Int J Pharm, 2017. 517(1-2): p. 157-167.
35. Beaumont, K.A., N. Mohana-Kumaran, and N.K. Haass, Modeling Melanoma In Vitro and In Vivo. Healthcare (Basel), 2013. 2(1): p. 27-46.
36. Giavazzi, R. and A. Decio, Syngeneic murine metastasis models: B16 melanoma. Methods Mol Biol, 2014. 1070: p. 131-40.
37. Overwijk, W.W. and N.P. Restifo, B16 as a mouse model for human melanoma. Curr Protoc Immunol, 2001. Chapter 20: p. Unit 20 1.
38. Abel, E.L., et al., Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc, 2009. 4(9): p. 1350-62.
39. Sharma, J. and P.K. Goyal, Chemoprevention of chemical-induced skin cancer by Panax ginseng root extract. J Ginseng Res, 2015. 39(3): p. 265-73.